Blood Activation Processes and Anticoagulant Coatings
HEMOCOMPATIBLE INTERFACES
Dr. Manfred Maitz, researcher
Dr. Claudia Sperling, researcher
Dr. Marion Fischer, researcher
Martina Franke, technician
Monique Marx, technician
Development of new strategies for anticoagulant coatings based on the exploration of surface - blood interactions
Hemocompatibility is a prerequisite for materials used in biomedical products like cardiovascular implants, catheters and medical membranes as otherwise surface initiated coagulation processes and immune reactions occur.
The systematic analyses of blood activation processes using fresh human whole blood and well defined model substrates expands the detailed knowledge of surface initiated blood activation on a quasi molecular level.
This competence supports the functionalization of surfaces utilizing synthetic or naturally occurring inhibitors for immobilization aiming at strategies for new anticoagulant coatings.
Blood Activation Processes
The goal of our work is the definition of a hemocompatible profile for biomaterial surfaces leading to knowledge based design of
hemocompatible material surfaces.
Biomaterials directly contacting human blood induce reactions of blood proteins and cells. The intensity and type of these reactions depends on biomaterials surface characteristics like roughness, charge, hydrophilicity and domain structure. There are several hypothesises on initial events of the blood-materials interaction yet due to the complexity of the reactions lots of questions remain unanswered.
The analysis of interactions of materials with blood components relies on the use of a realistic incubation set-up combined with well defined parameters as well as on surfaces which enable a direct correlation between reactions and surface characteristics.
Our in house designed reactors enable the incubation of a variety of material types with fresh whole human blood under flowing or non flowing conditions. The analysis of reactions of the incubated blood and the corresponding surfaces encompasses a wide range of analytical methods. Additionally several methods following surface incubation with different blood fractions like blood plasma, cells or proteins aid in the trial to get a whole picture of blood surface interactions.
The incubated surfaces are well defined models like self-assembled monolayer (SAM) exposing a variety of different functional groups and combinations of the latter to scrutinize fundamental hypothesises on initial events of the blood-materials interaction. Additionally polymer films using poly(octadecene (or alternatively ethylene- or propylene-) alt maleic anhydride) copolymers are used.
Anticoagulant Coatings
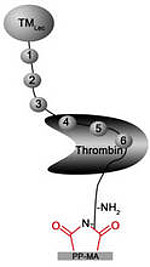
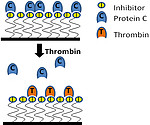
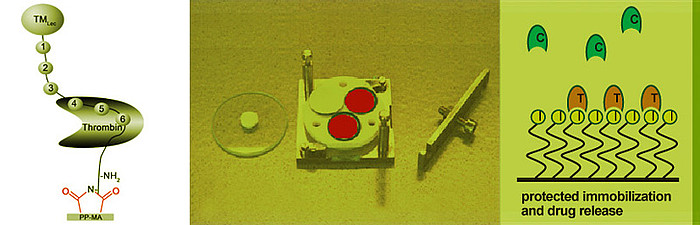
Thrombin is a key factor of the coagulation cascade and it is a main target for the physiological and medical coagulation regulation. Therefore we have chosen it also as a main target for our thromboresistant surface modifications in the following concepts:
Anticoagulant surfaces by immobilization of biomolecules
Biomolecules, which prevent blood coagulation in the blood vessels, such as the proteins thrombomodulin and activated protein C (APC) or polysaccharides like heparin, have been grafted to surfaces. Such molecules are highly effective but generally expensive and not very stable at sterilization, storage and in vivo.
One strategy to overcome these problems was grafting of polysaccharides like heparin and modified mono-, oligo- and polysaccharides, being more resistant to environmental influences.
Immobilized synthetic coagulation inhibitors
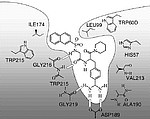
The binding pocket of the coagulation factor thrombin with its reactive centre is well characterized and inhibitor molecules with improved affinity to the reactive site were in house designed and synthesized. Besides this, commercially available inhibitor molecules are investigated. Such small molecules have the advantage of higher stability and lower costs than the biomolecules.
Bioresponsive anticoagulant systems
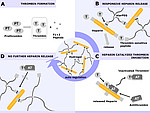
Anticoagulant surfaces require activity not only on the surface but also in the fluid phase in the sense of a release system. This ideally should be adjusted to the actual coagulation situation to avoid overdosage and preterm exhaustion. This was achieved by a hydrogel formed of the anticoagulant heparin and a four-armed PEG molecule using thrombin cleavable peptides as linker. Sufficient thrombin concentrations for cleavage of the linker are available only during blood clotting. The following release of heparin into the liquid phase disrupts further coagulation processes.
A second feedback system based on the structural similarities of thrombin and the anticoagulant Activated Protein C (APC) is under development. Both proteins have affinity to similar reversible small inhibitor molecules. Inhibitor molecules with such ambiguous affinity are immobilized to a surface and loaded with APC. High thrombin concentration during coagulation processes replaces the APC from the inhibitor. The pro-coagulant thrombin activity is quenched and the anticoagulant activity of APC becomes available.
Selected publications
Blood activation processes on biomaterial surfaces
- Herklotz, M.; Prewitz, M.C.; Bidan, C.M.; Dunlop, J.W.C.; Fratzl, P.; Werner, C.:
Availability of extracellular matrix biopolymers and differentiation state of human mesenchymal stem cells determine three-dimensional tissue-like growth in vitro. Biomaterials 60 (2015) 121-129 - Fischer, M.; Vahdatzadeh, M.; Konradi, R.; Friedrichs, J.; Maitz, M.; Freudenberg, U.; Werner, C.:
Multilayer hydrogel coatings to combine hemocompatibility and antimicrobial activity. Biomaterials 56 (2015) 198-205 - Fischer, M.; Sperling, C.; Tengvall, P.; Werner C.:
The ability of surface characteristics of materials to trigger leukocyte tissue factor expression. Biomaterials 31 (2010) 2498-507 - Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C.:
Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 30 (2009) 4447-56. - Sperling, C.; Maitz, M.F.; Talkenberger, S.; Gouzy, M.F.; Groth, T.; Werner, C.:
In vitro blood reactivity to hydroxylated and non-hydroxylated polymer surfaces. Biomaterials 28 (2007) 3617-25
Blood Incubation set-up
- Streller, U.; Sperling, C.; Hübner, J.; Hanke, R.; Werner, C.:
Design and Evaluation of Novel Blood Incubation Systems for In Vitro Hemocompatibility Assesment of Planar Solid Surfaces. Journal of Biomedical Materials Research 66b (2003) 379-390
Blood protein adsorption
- Fischer, M.; Baptista, C.P.; Gonçalves, I.C.; Ratner, B.D.; Sperling, C.; Werner, C.; Martins, C.L.; Barbosa, M.A.:
The effect of octadecyl chain immobilization on the hemocompatibility of poly (2-hydroxyethylmethacrylate). Biomaterials 33 (2012) 7677-7685 - Maitz, M.F.; Teichmann, J.; Sperling, C.; Werner, C.:
Surface endotoxin contamination and hemocompatibility evaluation of materials. J Biomed Mater Res B Appl Biomater 90 (2009) 18-25
Anticoagulant coatings
- Maitz, M.:
Applications of synthetic polymers in clinical medicine. Biosurface and Biotribology 1 (2015) 161-176 - Seib, F.P.; Herklotz, M.; Burke, K.A.; Maitz, M.F.; Werner, C.; Kaplan, D.L.:
Multifunctional silk-heparin biomaterials for vascular tissue engineering applications. Biomaterials 35 (2014) 83-91 - Maitz, M.F.; Freudenberg, U.; Tsurkan, M.V.; Fischer, M.; Beyrich, T.; Werner, C.:
Bio-responsive polymer hydrogels homeostatically regulate blood coagulation. Nature Communications 4 (2013) article number: 2168 - Maitz, M.F.; Sperling, C.; Werner, C.:
Immobilization of the irreversible thrombin inhibitor D-Phe-Pro-Arg-chloromethylketone (PPACK) a concept for hemocompatible surfaces?. J Biomed Mater Res A 94A (2010) 905-912 - Gouzy, M.F.; Sperling, C.; Salchert, K.; Pompe, T.; Rauwolf, C.; Werner, C.:
Benzamidine-based coatings: Implication of inhibitor structure on the inhibition of coagulation enzymes in solution and in vitro hemocompatibility assessment. Biointerphases 1 (2006) 146-155 - Sperling, C.; Salchert, K.; Streller, U.; Werner, C.:
Covalently immobilized thrombomodulin inhibits coagulation and complement activation of artificial surfaces in vitro. Biomaterials 25 (2004) 5101-5113
Specific instrumentation
- Blood incubation set-up: in-house developed instrumentation to analyze in vitro the interaction of material surfaces with fresh human whole blood under static and flow conditions
- Blood cell counter (Coulter AcT diff)
- Critical point dryer CPD030 (Bal-Tec)
- Fluorescent Image Analyzer FLA-5100 (Fujifilm) for fluorescent, chemiluminescent and radioactive samples and colorimetric applications
- Syringe pumps (kd scientific and Harvard Apparatus GmbH) for blood incubation using directed flow
- Pipetting robot (Tecan) for ELISA
- Flow cytometer FACS Calibur (Becton Dickinson)

