Headline
Technology Platform & Facilities
- Automated Synthesis of Bioactives, Polymer Conjugates and Hydrogel Networks
-
Peptide synthesis
Large scale solid phase peptide synthesis is applied to produce customized peptide sequences serving, e.g., as enzymatically cleavable linker-units or ligands of cell adhesion receptors. Automated supply of several resins and microwave induced synthesis allow for high throughput peptide screening and purification. Characterization of peptides is based on preparative HPLC, HPLC-ESI-TOF and MALDI-TOF.
Automated synthesis of polymer conjugates
A high-throughput synthesis robot (Biosynthesizer, BSyS 3.1) is used to fabricate functional polymer conjugates at high quality standards. Tailor-made synthesis of various conjugates is achieved by solid feedstock handling, temperature- and pH-control, optionally under inert conditions.
Hydrogel formation
Different covalent and non-covalent crosslinking schemes are used to convert functional polymer-peptide-conjugates into hydrogel networks by
- 2D and 3D (bio)printing to produce hydrogel (optionally cell-laden) arrays
- (Nano-Plotter NP2.1 and BioScaffolder, BS3.1) (see section array fabrication),
- droplet-based microfluidics for the generation of microparticles (microgels) and
- automated fluid handling (Fluent 480) to produce gel libraries in high throughput 384 well plate formats (optionally incorporating living cells).
SELECTED REFERENCES
Tsurkan M.V., Chwalek K., Prokoph S, Zieris A., Levental K.R., Freudenberg U., Werner C. Defined polymer-peptide conjugates to form cell-instructive starPEG-heparin matrices in situ. Advanced Materials 25(18): 2606-10 (2013)
Wieduwild R., Tsurkan M., Chwalek K., Murawala P., Nowak M., Freudenberg U., Neinhuis C., Werner C., Zhang Y. Minimal peptide motif for non-covalent peptide-heparin hydrogels. Journal of the American Chemical Society 135(8): 2919-22 (2013)
- Array Fabrication and Characterization
-
3D Printing
Multi-component inkjet printing methods are developed and applied for the formation of cell-instructive (optionally cell-laden) hydrogels with spatially varying mechanical and biomolecular properties (Nano-Plotter NP2.1, BioScaffolder BS3.1).
Array fabrication
3D inkjet printing (Nano-Plotter NP2.1, BioScaffolder, BS3.1) and robot-assisted fluid handling methods (TECAN Fluent 480) are applied to fabricate combinatorial hydrogel libraries. 2D contact printing techniques (Pin-Tool on Nano-Plotter and microcontact printer µCP 4.1) are used to create chemically micropatterned substrates.
Soft lithography
Soft lithography is applied to create microfluidic devices and micropatterned structures to control bioadhesion and enable biomechanical experimentation on various prokaryotic and eukaryotic species. The technique allows fast, repetitive and reproducible prototyping of master designs with different polymers.
Array characterization
Optical-3D characterization is accomplished using state-of-the-art spinning disc confocal microscopy (Dragonfly High Speed Confocal). Spatially-resolved mechanical properties are acquired by atomic force microscopy (AFM)-based colloidal probe measurements. Time of flight secondary ion mass spectrometry (ToF-SIMS) is used for chemical surface analysis, depth profiling and 3D imaging.

Array fabrication using multi-component bioprinting Copyright Sven Döring - Thin Film Preparation, surface modification and characterization
-
Atomic force microscope Polymer thin films are prepared under clean room conditions by spin- and dip-coating techniques. Thin films or polymer surfaces can be modified and/or structured by low pressure plasma treatments (MicroSys, Roth&Rau). Surface and interfacial properties are analyzed with respect to composition and chemical structure (x-ray photoelectron spectroscopy [XPS];
- time-of-flight secondary ion mass spectrometry [ToF-SIMS];
- ATR-FTIR topography (AFM; scanning electron microscopy (SEM) (FEI XL 30)),
- adsorbed mass (quartz crystal microbalance with dissipation monitoring [QCM-D]),
- (visco)elastic properties (QCM-D; AFM)
- surface charge (electrokinetic streaming potential/current measurements with in house developed microslit electrokinetic set-up (MES ZetaSCIENCE)) and
- layer thickness (QCM-D; ellipsometry; AFM).
- Analysis of Biomolecular Binding, Biomolecular Adsorption and cell-material Interactions
-
We apply surface plasmon resonance, microscale thermophoresis, Multiplex-ELISA, and radiolabeling techniques (iodine) to analyze molecular binding processes, e.g. to quantify interactions between morphogens and glycosaminoglycans. We investigate single, sequential and competitive protein adsorption at solid/liquid interfaces by using ellipsometry, QCM-D, radioisotope methods, immunostaining/fluorescence spectroscopy/microscopy, immunosorbent assays and AFM. Protein displacement in complex mixtures is analyzed using customized fluorescence labeling techniques. AFM-based single-cell force spectroscopy is utilized to quantify cellular interaction forces in near-physiological conditions with unrivaled spatial and temporal control of cells, as well as highly quantitative force actuation and force measurement that is sufficiently sensitive to characterize the interaction of single molecules.
Immunostaining and state-of-the-art spinning disc confocal microscopy (Dragonfly High Speed Confocal) is used to characterize morphology and fate decisions of 3D-cell cultures. Cryosectioning and resin embedding techniques are used to access volume samples. SEM is used to study the morphology of cells on material surfaces or in 3D (-hydrogel or extracellular matrix) environments. Wetting, swelling and related structural changes of biological samples (fibres, plant and animal samples) can be studied in situ using the environmental mode of scanning electron microscopy (ESEM).
- Cell and Tissue Culture
-
Primary, immortalized, as well as reprogrammed cells from several species (mouse, human) are grown in single cell-, co- and organoid-cultures, with or without exposure to functional biomaterials, e.g. for cytotoxicity screening of materials or for creating in vitro tissue or disease models.
Cell culture is performed in clean room facilities or cabinets under the safety level S2. Using multicolor-FACS analysis (LSR Fortessa; MACS Quant) we perform
Acquiring data at the spinning disk confocal simultaneous multiparametric analyses and quantifications of biomarker expression. To determine cell morphology and fate decisions, confocal imaging (Leica TCS SP5, Andor Dragonfly spinning disk confocal) is applied. Time-lapse microscopy for dynamic measurements or standard metabolom analysis techniques allowing for parallelization of samples are routinely used to analyze our cultures. Multiplex systems are applied to identify the cellular secretom (i.e. to detect multiple morphogens simultaneously).

Preparing the pipetting robot - Bacterial Adhesion and Biofilm Formation
-
We study the initial adhesion of bacteria and the subsequent biofilm formation on engineered surfaces to deploy and design strategies to avoid or control bacterial adhesion. Quantification of bacterial adhesion is performed under S2 safety level conditions using fluorescence tagging or staining. We exploit soft lithography to pattern substrates and template bacterial shape to study bacterial adhesion of Gram-positive and Gram-negative pathogenic species.
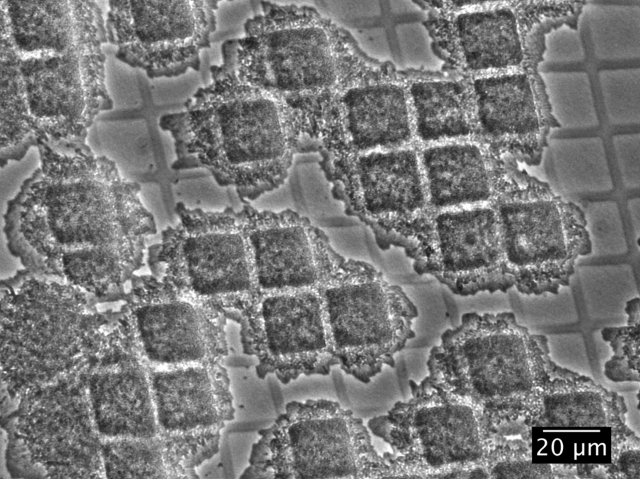
Biofilm development on a structured substrate - Hemocompatibility Assessment
-
The hemocompatibility of materials is tested by incubation experiments with human whole blood under flowing or non-flowing conditions, analysing hemostatic and inflammatory processes. Surfaces and blood samples are analysed after incubation to conclude on activation of coagulation, platelets, immune reactions and hemolysis. Cell numbers are determined by a differential blood cell counter while multicolor-flow cytometric analysis is used for the detection of specific activation signals on the surface of blood cells. The analysis of activation molecules in plasma by ELISA is assisted by a liquid handling workstation.
Selected References
Sperling C., Maitz M.F., Werner C. Test methods for hemocompatibility of biomaterials. in: C.A. Siedlecki (Ed.), Hemocompatibility of Biomaterials for Clinical Applications. Elsevier, Amsterdam, pp. 77-104 (2017).
Braune S., Sperling C., Maitz M.F., Steinseifer U., Clauser J., Hiebl B., Krajewski S., Wendel H.P., Jung F. Evaluation of platelet adhesion and activation on polymers: Round-robin study to assess inter-center variability. Colloids and Surfaces B: Biointerfaces 158, 416-422 (2017).
Streller U., Sperling C., Hübner J., Hanke R., Werner C. Design and evaluation of novel blood incubation systems for in vitro hemocompatibility assessment of planar solid surfaces. Journal of Biomedical Materials Research 66B(1), 379-390 (2003).

Fresh whole blood from human donors is analyzed before the incubation with the material surfaces 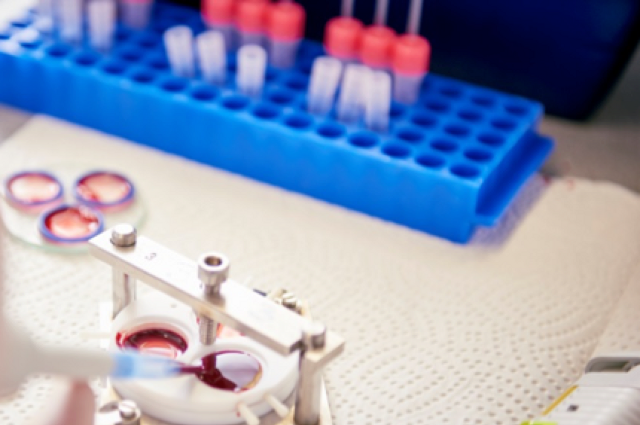
After the incubation blood is removed from the custom made incubation chambers and analysed for parameters of hemostasis and inflammation while surfaces are rinsed and prepared for surface analysis 
Specific surface activation markers on blood cells are detected using flow cytometry - HTS-Facility: High-Throughput screenings, application & translation of research
-
Our Aim
We focus on the automated preparation, maintenance and characterization of hydrogel-based human tissue and disease models for high throughput screening experimentation (HTS). State-of-the-art tools are available to perform screenings in a highly parallelized manner, for example testing several compounds at different concentrations to assess their toxicity on healthy tissue models, or their effectiveness in treating diseased cells.
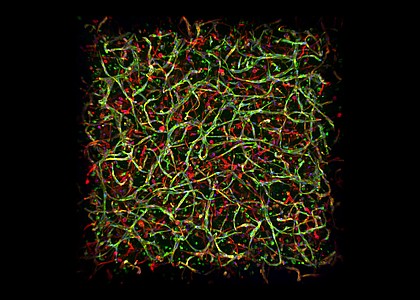
Low-volume 3D culture of endothelial cells (HUVECs, green) and supporting stromal cells (MSCs, red). Automated Cell Culture and Analysis
Janus G3
Combines the versatility of an 8-channel pipetting arm with the throughput of a 96-channel arm for precise production, maintenance and fluorescent staining of hundreds of individual cultures per assay plate.
TECAN Fluent 480
An additional liquid handling system complements the Janus G3 for an increased throughput in the automated production of hydrogels and 3D cell cultures within a 384-well plate, as well as serial dilutions of screening compounds and sample preparation.
Opera Phenix
Directly connected to the Janus G3, this fully automated spinning disc confocal microscope is designed for the rapid imaging and analysis of hundreds of individual 3D cultures per assay. An additional pipetting arm allows the addition of compounds to the plate while imaging to capture fast kinetic processes.DESIGN OF EXPERIMENTS (DOE)
Employed in research fields ranging from drug discovery to process engineering, the statistical tool DOE enables the systematic and efficient characterization of multiparametric models. It allows to precisely characterize how each parameter contributes to the model, while minimizing the number of parameter combinations actually tested. DOE plays a key role in identifying optimal culturing protocols for our 3D hydrogel-based tissue and disease models.

TECAN Fluent 480 liquid handling robot.
Image credit: Krishna Gupta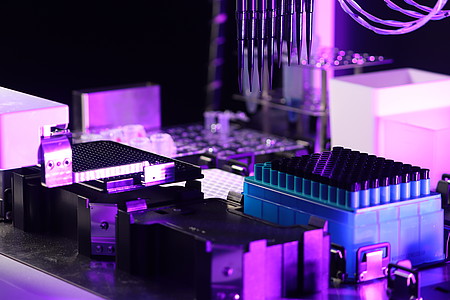
HTS platform connecting a Janus G3 liquid handling robot, an Opera
Phenix Plus spinning disc confocal microscope and a cell culture
incubator, allowing the fully automated production and processing
of 3D cultures in 384-well plate format.
Image credit: Krishna Gupta
