Dr. Jens Friedrichs, researcher
Dr. Ralf Helbig, researcher
Julia Nickerl, PhD student
Nicole Träber, PhD student
Adhesive interactions of cells with (bio)materials trigger a multitude of desired, but also undesired processes. On one hand, these interactions can induce signalling pathways that are involved in regulating important cellular processes including cell migration, gene expression, cell survival, tissue organization, and differentiation. On the other hand, the deposition of microorganisms and their extracellular polymeric substrates on material surfaces can lead to significant health risks and financial losses in medical, marine and industrial fields.
We focus on the development of faithfully designed, biomimetic (bio)polymer matrices to control and guide adhesive interactions. Furthermore, we apply atomic-force microscopy-based methods to characterize the structure and function of these matrices.
Basement membranes are specialized extracellular matrices that have important roles in cell attachment, migration, growth, and differentiation. Recent advancements in bioengineering result in a strong demand for bioartificial in vivo-like, basement membrane-mimicking environments. We reconstitute highly defined and immobilized bioartificial basement membrane-like matrices from combinations of recombinant extracellular matrix proteins using cell-produced basement membranes as blueprints. The bioartificial basement membrane-like matrices developed find application in advanced cell culture studies and in tissue engineering.
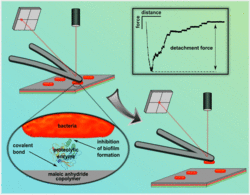
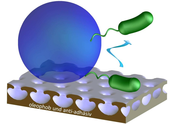
Adhesive interactions of cells with their environment can trigger a multitude of desired, but also undesired processes. An undesired, adhesion-associated process is the deposition of microorganisms and their extracellular polymeric substrates on man-made surfaces, so called biofouling or biofilm formation. This phenomenon can occur in an extremely wide range of situations, from the colonization of medical- and process devices to the production of ultra-pure, drinking and process water and the fouling of ship hulls, pipelines and reservoirs. In this context we focus on the development, characterization and application of well-characterized surfaces with antifouling properties. More precisely, we investigate the influence of biofilm-degrading enzymes, covalently immobilized onto maleic anhydride copolymer films, on the adhesion of major marine foulers and bacteria associated with hospital-acquired infections.
Natural surfaces often possess amazing shapes and structures that arouse interest for both basic research and commercial applications. A pertinent example is the skin of springtails (Collembola) that features mechanically stable, hierarchical, micro- to nanoscale structural elements that equips the animal with omniphobic, anti-adhesive properties. We focus on characterizing the skin of springtails and recapitulate their robust and effectively repellent surface characteristics. Furthermore, we deduce design principles for non-wetting and antifouling surfaces from the structural and chemical features of the skin.
- Theoretical models for wetting transition on complex shaped surface features
- Mimicking the omniphobic characteristics by polymer membranes
- Chemical characterization of the skin
- Bacterial adhesion tests on synthetic surfaces mimicking structure and chemistry of the springtail skin
Blood homeostasis is dependent on the numerous interactions between primitive blood cells and the different cell types present in the bone marrow as well as the extracellular matrix. We apply AFM-based single-cell force spectroscopy (SCFS) to quantify the interactions between hematopoietic stem and progenitor cells (HSPC) as well leukemic cells and (i) cell types present in the bone marrow (i.e. multipotent mesenchymal stromal cells [MSCs], osteoblasts and endothelial cells), (ii) a set of defined decellularized extracellular matrices (derived from MSC cultures and (iii) engineered (bio)polymer matrices)
- Reichert, D.; Friedrichs, J.; Ritter, S.; Käubler, T.; Werner, C.; Bornhäuser, M.; Corbeil, D.:
Phenotypic, morphological and adhesive differences of human hematopoietic progenitor cells cultured on murine versus human mesenchymal stromal cells. Scientific Reports 5, Article number: 15680 (2015) doi:10.1038/srep15680 - Prewitz, M.; Stißel, A.; Friedrichs, J.; Träber, N.; Vogler, S.; Bornhäuser, M.; Werner, C.:
Extracellular matrix deposition of bone marrow stroma enhanced by macromolecular crowding. Biomaterials 73 (2015) 60-69 - Hensel, R.; Neinhuis, C.; Werner, C.:
Omniphobic characteristics of animals in aqueous or temporarily flooded habitats and their applications. Chemical Society Reviews 2015, Doi:10.1039/c5cs00438a - Friedrichs, J.; Werner, C.:
Polymeric coatings to fight biofouling. In: Encyclopedia of Polymeric Nanomaterials: Antifouling and antimicrobial polymeric coatings/ed. by S. Kobayashi and K. Müllen. Springer Verlag Berlin, Heidelberg, 2015, 1944-1950 - Fischer, M.; Vahdatzadeh, M.; Konradi, R.; Friedrichs, J.; Maitz, M.; Freudenberg, U.; Werner, C.:
Multilayer hydrogel coatings to combine hemocompatibility and antimicrobial activity. Biomaterials 56 (2015) 198-205 - Bray, L.; Binner, M.; Holzheu, A.; Friedrichs, J.; Freudenberg, U.; Hutmacher, D.W.; Werner, C.:
Multi-parametric hydrogels support 3D in vitro bioengineered microenvironment models of tumour angiogenesis. Biomaterials 59 (2015) 609-620 - Hensel, R.; Finn, A.; Helbig, R.; Killge, S.; Braun, H.-G.; Werner, C.:
In situ experiments to reveal the role of surface feature sidewalls in the Cassie-Wenzel transition. Langmuir 30 (2014) 15162-15170 - Nickerl, J.;Tsurkan, M.; Hensel, R.; Neinhuis, C.; Werner, C.:
The multi-layered protective cuticle of Collembola: A chemical analysis. Journal of the Royal Society Interface 11 (2014) 20140619 - Schubert, R.; Strohmeyer, N.; Bharadwaj, M.; Ramanathan, S.P.; Krieg, M.; Friedrichs, J.; Franz, C.M.; Müller, D.J.:
Assay for characterizing the recovery of vertebrate cells for adhesion measurements by single-force spectroscopy. FEBS Letters 588 (2014) 3639-3648 - Welzel, P.; Friedrichs, J.; Grimmer, M.; Vogler, S.; Freudenberg, U.; Werner, C.:
Cryogel micromechanics unraveled by atomic force microscopy-based nanoindentation. Advanced Healthcare Materials 3 (2014) 1849-1853 - Hensel, R. et al.:
Biologically inspired omniphobic surfaces by reverse imprint lithography. Advanced Materials 26 (2014) 2029-2033 - Arnold, G.; Schade, E.; Schneider, Y.; Friedrichs, J.; Babick, F.; Werner, C.; Rohm, H.:
Influence of individual phospholipids on the physical properties of oil-based suspensions. Journal of thr American Oil Chemists' Society 91 (2014) 71-77 - Hensel, R.; Helbig, R.; Aland, S.; Voigt, A.; Neinhuis, C.; Werner, C.:
Tunable nano-replication to explore the omniphobic characteristics of springtail skin. NPG Asia Materials 5 (2013), e37 - Hensel, R.; Helbig, R.; Aland, S.; Braun, H.-G.; Voigt, A.; Neinhuis, C.; Werner, C.:
Wetting resistance at its topographical limit The benefit of mushroom and serif T structures. Langmuir 29 (2013) 1100-1112 - Prewitz, M. et al.:
Tightly anchored tissue-mimetic matrices as instructive stem cell microenvironments. Nature Methods 10 (2013) 788-794 - Teräväinen, T.P. et al.:
∝V-Integrins are required for mechanotransduction in MDCK epithelial cells. PLoS ONE 8 (2013) e71485 - Friedrichs, J.; Werner, C.; Müller, D.J.:
Quantifying cellular adhesion to covalently immobilized extracellular matrix proteins by single-cell force spectroscopy. Methods Mol Biol 1046 (2013) 19-37 - Friedrichs, J.; Zieris, A.; Prokoph, S.; Werner, C.:
Quantifying the effect of covalently immobilized enzymes on biofilm formation by atomic force microscopy-based single-cell force spectroscopy. Macromolecular Rapid Communications 33 (2012) 1453-1458 - Helbig, R.; Nickerl, J.; Neinhuis, C.; Werner, C.:
Smart skin patterns protect springtails. PLoS ONE 6 (2011) e25105 - Friedrichs, J.; Helenius, J.; Müller, D.J.:
Quantifying cellular adhesion to extracellular matrix components by single-cell force spectroscopy. Nature Protocols 5 (2010) 1353-1361 - Friedrichs, J.; Manninen, A.; Müller, D.J.; Helenius, J.
Galectin-3 regulations integrin alpha2beta1-mediated adhesion to collagen -I und -IV. J Biol Chem 283 (2008) 32264-32272 - Taubenberger, A. et al.:
Revealing early steps of 2 1 integrin-mediated adhesion to collagen type I by using single-force spectroscopy. Mol Biol Cell 18 (2007) 1634-1644
- Nanowizard I atomic force microscope (JPK Instruments) mounted on an inverted optical microscope (Zeiss Axio Observer. A1)
- Nanowizard II atomic force microscope (JPK Instruments) mounted on an inverted optical microscope (Zeiss Axio Observer.D1) equipped with a CellHesion Module (extended z-range of 100 µm), temperature-controlled sample stages (BioCell, PetriDish Heater and a Heating-Cooling Stage), a kelvin probe and scanning capacitance microscopy module and a top-view optic.
Examples of application: - Polymer and thin film imaging
- Imaging of functionalized surfaces
- (Live) cell imaging
- Single-cell force spectroscopy (Cell adhesion)
- Nanoindentation measurements on biological and non-biological samples
- Binding studies e.g. antibody/antigen, receptor/ligand
- UV-Imprint lithography (PFPEdma templates)
- Soft lithography (PDMS templates)
- Optical Surface Analysis (Nanofocus μsurf explorer)
- Contact Angle System (Dataphysics OCA 30)